Science Study Guide for the TEAS
Page 2
Human Anatomy and Physiology: The Respiratory and Cardiovascular Systems
Anatomy and Physiology of the Respiratory System
The function of the respiratory system is to bring oxygen into the body and to expel carbon dioxide from the body. It consists of tubes that transport air into the body and air sacs where these gases are exchanged in the bloodstream. The respiratory system also filters out particles from the air, helps produce sound, has a role in the sense of smell, and controls the temperature and humidity of the air entering the lungs.
Structure
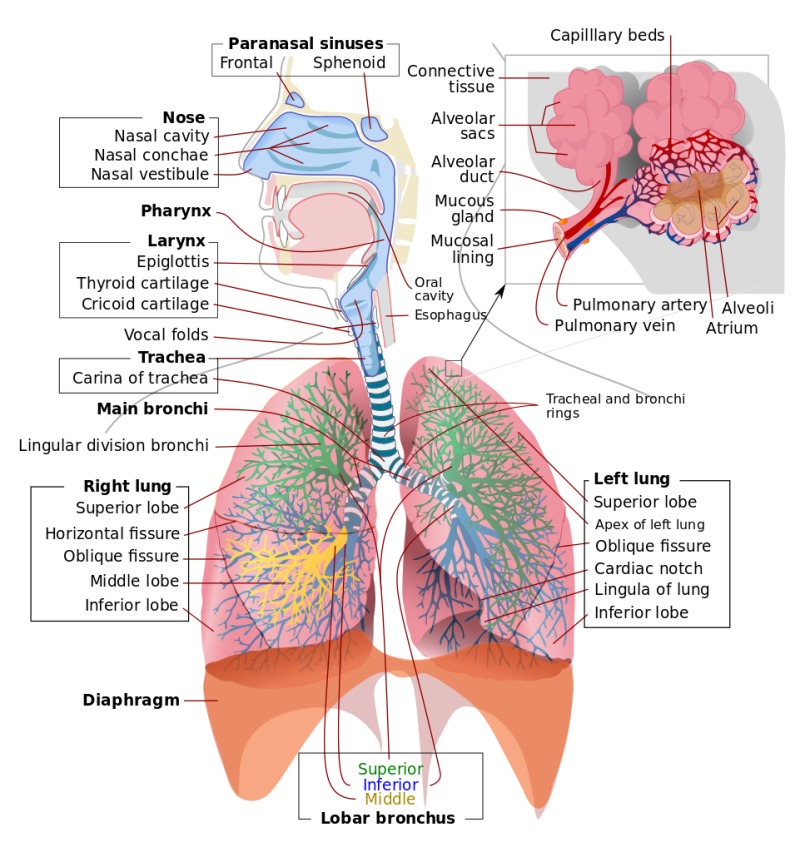
Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK459258/bin/Respiratory_system_complete_en.svg.jpg Taken from: Anatomy, Airway. Copyright 2022, StatPearls Publishing LLC. Creative Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/)
The respiratory system includes the nose, throat, and lungs, as well as the trachea, bronchi, and diaphragm. The respiratory system can be divided into the upper respiratory tract (nose, sinuses, pharynx, and larynx) and the lower respiratory tract (trachea, bronchi, and lungs).
Interaction with the Cardiovascular System
Although the respiratory system is typically described as the mouth and lungs, respiration is more than just breathing. It’s the process of using oxygen to break down food for energy. The respiratory system is responsible for external respiration, or getting oxygen from the external environment into the body. Once the oxygen enters the lungs, oxygen diffuses into the blood. Internal respiration involves the transport of oxygen to all the tissues and cells in the body through the blood. Finally, cellular respiration occurs inside where oxygen is used to break glucose molecules down for energy. The blood then transports the waste product, carbon dioxide, back to the lungs where it is exhaled. The respiratory and cardiovascular system are tightly intertwined.
Function
The function of the respiratory system is to receive and deliver oxygen and remove carbon dioxide. The diaphragm is a sheet of muscle that enables breathing. The intercostal muscles and accessory muscles can also be used to enable greater expansion. Inside the lungs, oxygen and carbon dioxide are exchanged between the air and blood via diffusion. This process occurs in the alveoli. The rate at which gas enters or leaves the lungs is called ventilation and is controlled by the autonomic nervous system, specifically by the medulla oblongata and pons. If the level of carbon dioxide in the blood increases, this is detected by receptors in the aorta, carotid artery, and medulla. A signal is then sent to increase the rate of breathing to remove excess \(\text{CO}_2\).
Factors Affecting the Respiratory System
The effectiveness of the respiratory system can change due to internal or external influences. These changes may be lifelong or short-term. Anything that restricts the passages through which air moves, alters the composition of the air reaching the lungs, or changes the capacity of the lungs can cause problems.
Genetic
Genetic conditions such as cystic fibrosis can clog the airways with mucus. Conditions with genetic and environmental influences include allergies and asthma.
Environmental
Intense and/or long-term exposure to pollutants, such as asbestos, particulates, and smog, can damage the airway and lungs. Smoking and the inhalation of other chemicals can lead to damaged airways as well. COPD, emphysema, mesothelioma, and lung cancer are some conditions caused by exposure to pollutants and chemicals.
Pathogens
Pathogens that affect the airway and lungs include viral and bacterial pneumonia, tuberculosis, the common cold, the flu, SARS, and COVID-19. Conditions such as bronchitis, an inflammation of the bronchial tubes, can develop due to viruses such as the cold or flu.
Anatomy and Physiology of the Cardiovascular System
The cardiovascular system, also called the circulatory system, delivers oxygen and nutrients to the cells in the body, and carries away waste products for removal. It is one of the most vital systems because, without a steady supply of oxygen and nutrients, cells can quickly die. That is why diseases that affect this system can be life threatening.
Structure
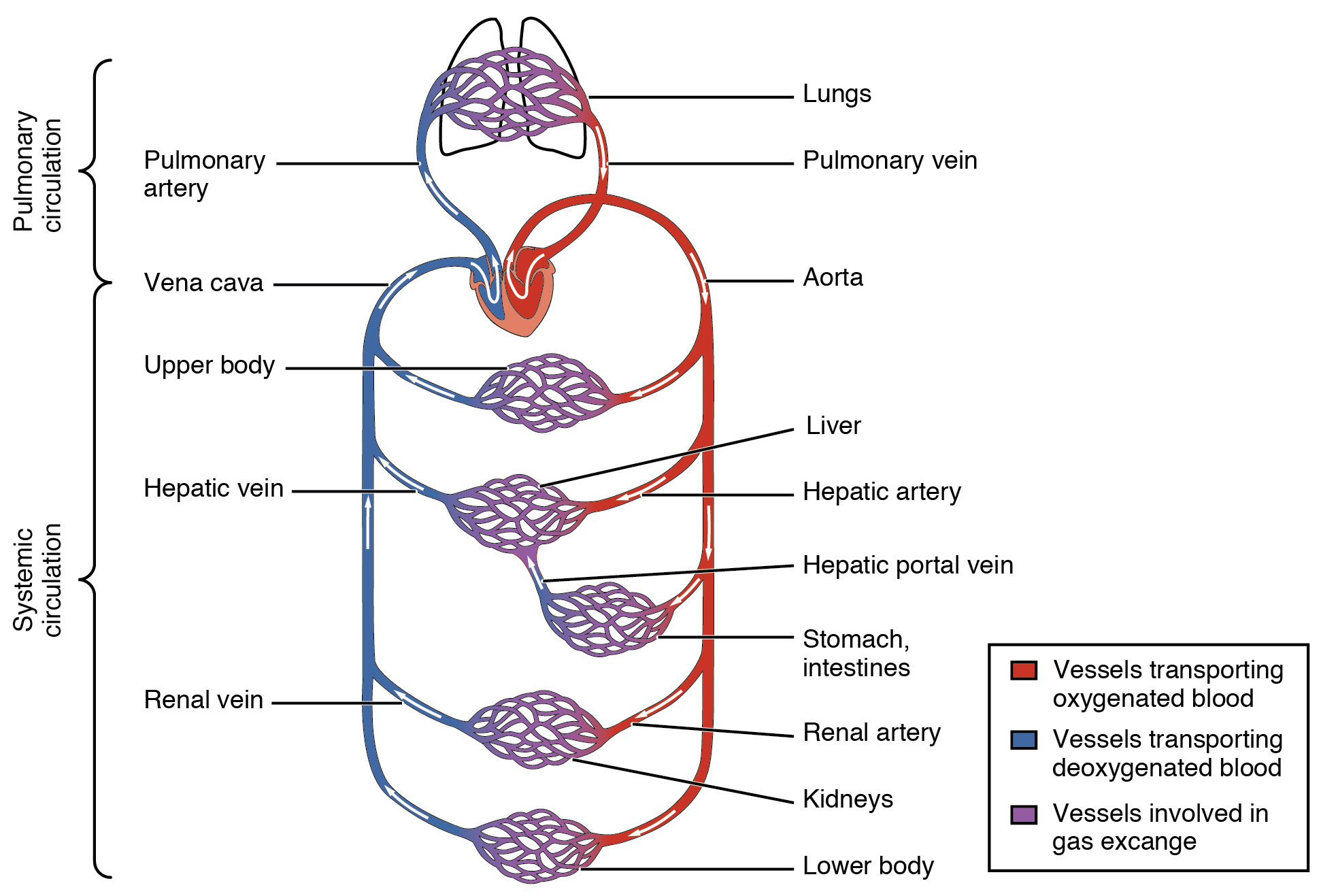
Retrieved from: https://openstax.org/apps/archive/20220815.182343/resources/8b8b0fbc51634078bbcf93b75762143a4a80cd47
The circulatory, or cardiovascular, system is composed of the heart, blood vessels, and blood. The blood vessels that carry blood away from the heart are the arteries, and the vessels that carry blood toward the heart are veins. The blood carries gases, wastes, and nutrients to and from cells. These are exchanged in small vessels called capillaries. The red blood cells that carry oxygen are generated in the bone marrow; since they only last about four months, they are continually being replaced by new cells.
Function
The function of this system is to transport oxygen and nutrients around the body. It is important to understand the anatomy of blood, blood cells, the circulatory system, the heart, and the cardiac cycle, as well as issues regarding these parts and systems.
Blood
The blood consists of red blood cells, white blood cells, platelets, and dissolved minerals. The red and white blood cells are generated in the bone marrow. Oxygen binds to the hemoglobin found in red blood cells. White blood cells are involved in the body’s immune response. Platelets are small structures that heal any ruptured vessels by clotting together.
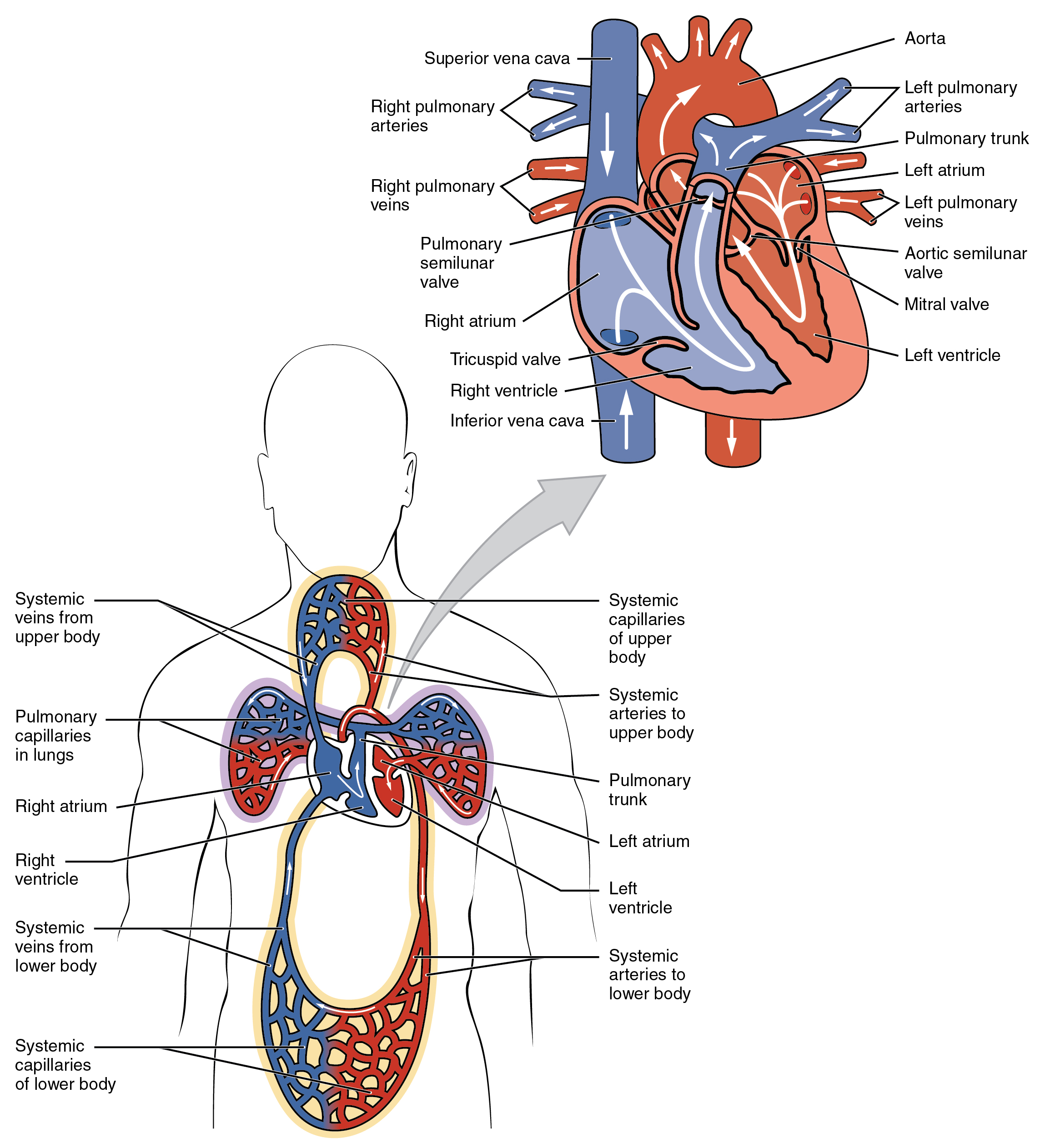
Retrieved from: https://openstax.org/books/anatomy-and-physiology/pages/19-1-heart-anatomy
The Heart
The heart is a muscular organ about the size of a fist. It has four chambers. The atria are the upper chambers that receive blood. The right atrium receives blood returning from the body, and the left atrium receives blood returning from the lungs. The blood in each chamber passes through a valve into the lower chambers, the ventricles. The ventricles are more muscular, and it is their job to pump blood out of the heart to the lungs and the rest of the body.
Blood Flow
There are two circuits through which blood travels: pulmonary and systemic. The pulmonary circuit carries deoxygenated blood away from the heart to the lungs, where carbon dioxide is removed and oxygen binds to the hemoglobin in red blood cells. The oxygenated blood is then taken back to the heart, where it can be circulated by the systemic circuit to deliver oxygenated blood to the rest of the body. The blood also transports nutrients and waste.
Measuring Cardiovascular Function
The pulse rate is the number of heartbeats per minute, and a normal pulse rate for a healthy adult should fall between 60 and 100 beats per minute. There are two types of blood pressure: systolic and diastolic. Systolic pressure is the pressure inside the arteries when the heart contracts and should be less than 120 mm Hg. Diastolic pressure is the pressure in the arteries when the heart relaxes. Its normal value is under 80 mm Hg.
All Study Guides for the TEAS are now available as downloadable PDFs