Chemistry Study Guide for the HESI Exam
Page 1
General Information
While chemistry may not seem relevant to nursing, it’s more applicable than you might think. Nurses must understand the use of the medication they are providing, the conversions, and they even need to understand how the medications work.
Advancing your career into more specialized fields requires you to possess not only a basic understanding of chemistry, but perhaps organic chemistry, or physical chemistry as well.
Having a solid foundation in chemistry is key to understanding how certain drugs interact, which directly affects patient outcomes. Here are some basic chemistry concepts you should understand in order to do well on the Chemistry section of the HESI exam:
Units of Measurement
While most measurements in nursing use the metric system, you may need to be able to approximate the US and/or Imperial measurements as a comparison.
Volume
Volume is usually measured in milliliters (ml) or cubic centimeters (\(cm^3\)).
- 1 ml = 0.001 liter
- 1 ml = 1 \(cm^3\)
- For example: 5 L = 5000 ml = 5000 \(cm^3\)
For larger quantities, US gallons are used in the United States and Imperial gallons are used in the United Kingdom. Some helpful conversions are:
- 1 Imperial gallon = ~ 1.2 US gallons
- 1 US gallon = ~ 3.79 liters or 231 cubic inches
- 1 Imperial gallon = ~ 4.55 liters or ~ 277.42 cubic inches
- 1 liter = 2.11 US pints
Mass and Length
-
Mass is usually measured in grams (g) or kilograms (kg), where 1 kg = 1000 g.
- 1 kg = 2.2 pounds
- 1 pound = 16 ounces
-
1 stone = 14 pounds
-
Length is commonly measured in meters (m), centimeters (cm), or millimeters (mm).
- 1 inch = 2.54 cm
- 1 foot = 30.5 cm
- 1 yard = 91.44 cm
- 1 km = 0.621 miles
States of Matter
There are four fundamental states of matter―solid, liquid, gas, and plasma.
Solids are characterized by closely packed particles, held together by strong intermolecular forces to form a definite shape. Heating a solid to a temperature above its melting point transforms it into a liquid.
In liquids, the intermolecular forces are weaker, meaning the particles have more freedom of movement. A liquid can be turned into a gas by heating it to a temperature higher than its boiling point.
In a gas, the particles have enough kinetic energy to overcome the intermolecular forces and can move freely. Heating a gas to extremely high temperatures can produce plasma.
This causes the electrons in an atom to separate from the nuclei and plasma can be thought of as a cloud of free electrons and positively charged ions.
Atoms
Elements are made of atoms, and atoms are made of three types of particles―negatively charged electrons, positively charged protons, and neutral neutrons.
The protons and neutrons make up the nucleus of the atom. The nucleus has a very small diameter compared to the overall size of the atom, but it is where most of the mass is concentrated.
The electrons orbit the nucleus in shells and most of the volume of the atom is taken up by the free space between the nucleus and electrons.
An element’s nuclear symbol tells you how many electrons, protons, and neutrons make up each atom.
\[^A_ZX\]\(X\) is the element symbol.
\(A\) is the mass number, which tells you the total number of protons and neutrons in the nucleus.
\(Z\) is the atomic number, which is the number of protons in the nucleus.
For neutral atoms, the number of protons is equal to the number of electrons. The number of neutrons is \(A\)—\(Z\).
For example, a neutral atom of lithium represented by the nuclear symbol \(^7_3Li\) has three protons, three electrons, and four neutrons.
Ions have unequal numbers of protons and electrons. Positive ions have more protons than electrons and negative ions have more electrons than protons.
All atoms of the same element have the same number of protons. Isotopes are atoms of the same element but with different numbers of neutrons. For example, \(^{12}_{\;6}C\) and \(^{13}_{\;6}C\) are both isotopes of carbon.
Acids and Bases
The acidity or basicity of a chemical or solution can be measured using the pH scale.
- The pH scale ranges from \(\bf0\) to \(\bf14\) and a pH of \(\bf7\) is neutral.
- A solution with a pH lower than \(\bf7\) is classed as acidic.
- A solution with a pH higher than \(\bf7\) is classed as basic.
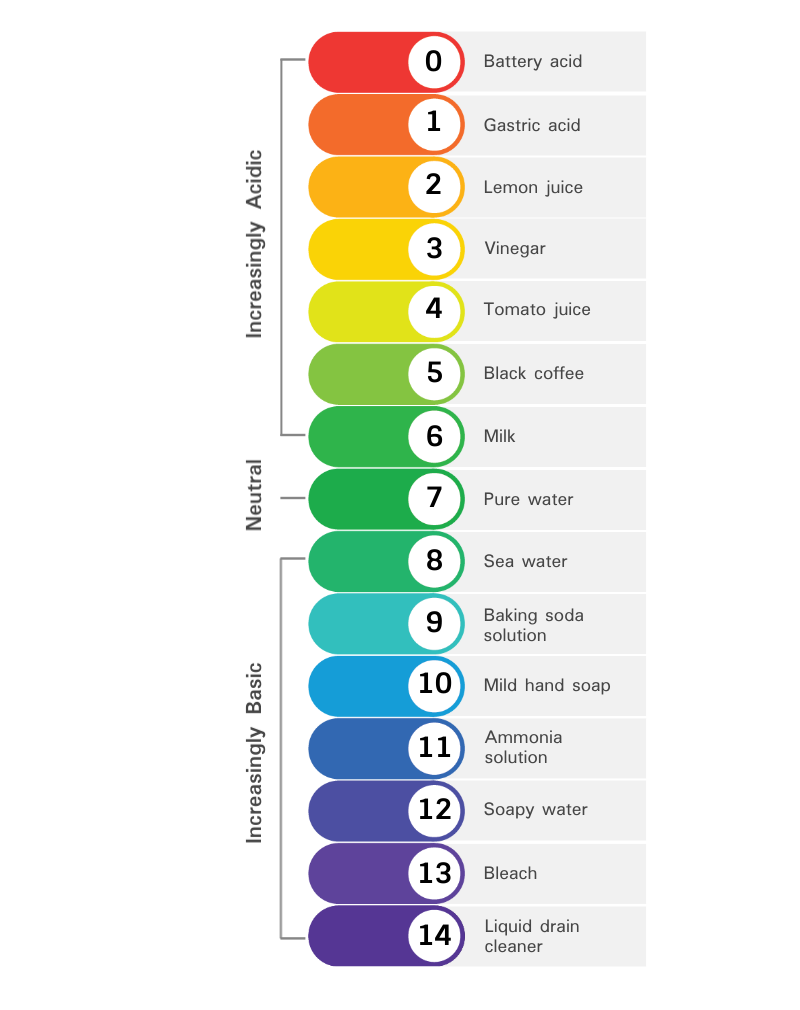
Hydrochloric acid (HCl), which is part of the acid that is found in the stomach, is a very strong acid with a pH of \(1\). Water is neutral, with a pH of \(7\). Sodium bicarbonate (\(NaHCO_3\)) has a pH of \(9\), making it a weak alkali. Sodium hydroxide (NaOH) is a very strong alkali and when concentrated can have a pH of \(14\).
The pH scale is logarithmic with a base of \(\bf10\). This means that each unit difference corresponds to a change of a factor of \(10\). For example, pH \(3\) is \(10\) times more acidic than pH \(4\) and \(1000\) times more acidic than pH \(6\).
Chemical Equations
Balanced equations should have the same number of each type of atom on both sides. To balance equations, you have to change the number of molecules of one or more compounds on either side of the equation. Balance the equation:
\[C_2H_6+O_2 ➡️ CO_2+2H_2O\]The first step is to work out the number of atoms on each side:
| Left | Right | |
| C = 2 | C = 1 | |
| H = 6 | H = 4 | |
| O = 2 | O = 3 |
The right side needs \(1\) more ion of \(C\) and \(2\) more ions of \(H\), so we can add \(1\) more \(CO_2\) compound and \(1\) more \(H_2O\) compound and the equation becomes:
\[C_2H_6+O_2 ➡️ 2CO_2+3H_2O\]| Left | Right | |
| C = 2 | C = 2 | |
| H = 6 | H = 6 | |
| O = 2 | O = 7 |
Because we have also added more atoms of \(O\) to the right, we now need five more atoms of \(O\) on the left to make \(7\) atoms in total. Add another \(2.5\) atoms of \(O_2\) to the left and the equation is balanced:
\[C_2H_6+3.5O_2 ➡️ 2CO_2+3H_2O\]| Left | Right | |
| C = 2 | C = 2 | |
| H = 6 | H = 6 | |
| O = 7 | O = 7 |
However, chemical equations typically do not include fractional coefficients, so let’s multiply the entire equation by \(2\) to eliminate the fraction, \(3.5\):
\[2(C_2H_6) + 7(O_2) ➡️ 4(CO_2) + 6(H_2O)\]Chemical Reactions
Particles in liquids and gases are constantly moving and colliding with each other. Under the right conditions, these particles can react. To start a reaction, the particles need to have a minimum amount of kinetic energy, known as the activation energy. This is the amount of energy required to break the bonds within each particle.
The rate of a reaction increases as temperature increases because the particles will have more kinetic energy. This means that they will be moving faster and colliding more often and be more likely to have enough energy to break the activation barrier. Increasing concentration also increases the rate of reaction because this will increase the number of particle collisions.
Catalysts can also be added to increase the reaction rate. These work by lowering the activation energy. In the following example, platinum is used as a catalyst in the reaction, which makes nitric acid from ammonia. Notice that platinum does not appear in the equation. This is because a catalyst is not consumed or changed during a chemical reaction.
\[NH_3+O_2 ➡️ HNO_3\]Oxidation and Reduction Reactions
Oxidation is the loss of electrons. Reduction is a gain of electrons. In redox (oxidation and reduction) reactions, oxidation and reduction occurs simultaneously.
In the reaction:
\[2Na + Cl_2 ➡️ 2Na+Cl\]sodium is oxidized because it loses an electron and chlorine is reduced because it gains an electron―sodium is the electron donor and chlorine is the electron acceptor.
All Study Guides for the HESI Exam are now available as downloadable PDFs