Isotonic, Hypotonic, and Hypertonic Solutions
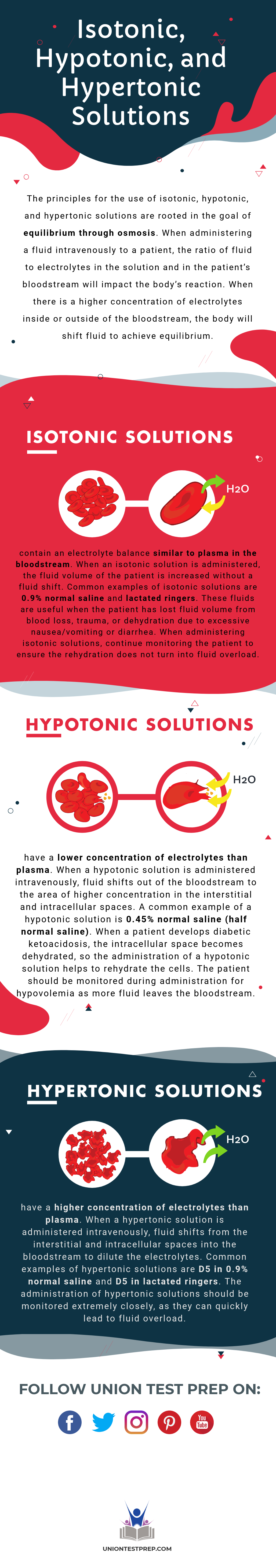
The principles for the use of isotonic, hypotonic, and hypertonic solutions are rooted in the goal of equilibrium through osmosis. When administering a fluid intravenously to a patient, the ratio of fluid to electrolytes in the solution and in the patient’s bloodstream will impact the body’s reaction. When there is a higher concentration of electrolytes inside or outside of the bloodstream, the body will shift fluid to achieve equilibrium.
Isotonic Solutions
Isotonic solutions contain an electrolyte balance similar to plasma in the bloodstream. When an isotonic solution is administered, the fluid volume of the patient is increased without a fluid shift. Common examples of isotonic solutions are 0.9% normal saline and lactated ringers. These fluids are useful when the patient has lost fluid volume from blood loss, trauma, or dehydration due to excessive nausea/vomiting or diarrhea. When administering isotonic solutions, continue monitoring the patient to ensure the rehydration does not turn into fluid overload.
Hypotonic Solutions
Hypotonic solutions have a lower concentration of electrolytes than plasma. When a hypotonic solution is administered intravenously, fluid shifts out of the bloodstream to the area of higher concentration in the interstitial and intracellular spaces. A common example of a hypotonic solution is 0.45% normal saline (half normal saline). When a patient develops diabetic ketoacidosis, the intracellular space becomes dehydrated, so the administration of a hypotonic solution helps to rehydrate the cells. The patient should be monitored during administration for hypovolemia as more fluid leaves the bloodstream.
Hypertonic Solutions
Hypertonic solutions have a higher concentration of electrolytes than plasma. When a hypertonic solution is administered intravenously, fluid shifts from the interstitial and intracellular spaces into the bloodstream to dilute the electrolytes. Common examples of hypertonic solutions are D5 in 0.9% normal saline and D5 in lactated ringers. The administration of hypertonic solutions should be monitored extremely closely, as they can quickly lead to fluid overload.
Keep Reading
Health Education Systems Incorporated Exam Blog
HESI Anatomy and Physiology Review: A Comprehensive Guide
As you prepare for the Health Education Systems, Inc (HESI) Admission A…

Health Education Systems Incorporated Exam Blog
How to Pass the HESI Exam: A Comprehensive Guide to Acing Your Nursing Entrance Exam
If you’re considering becoming a nurse, the HESI exam can be a crucial …

Health Education Systems Incorporated Exam Blog
The Complete Guide to HESI Exam Scores
Are you ready to become a nurse? Many nursing schools require entrance …